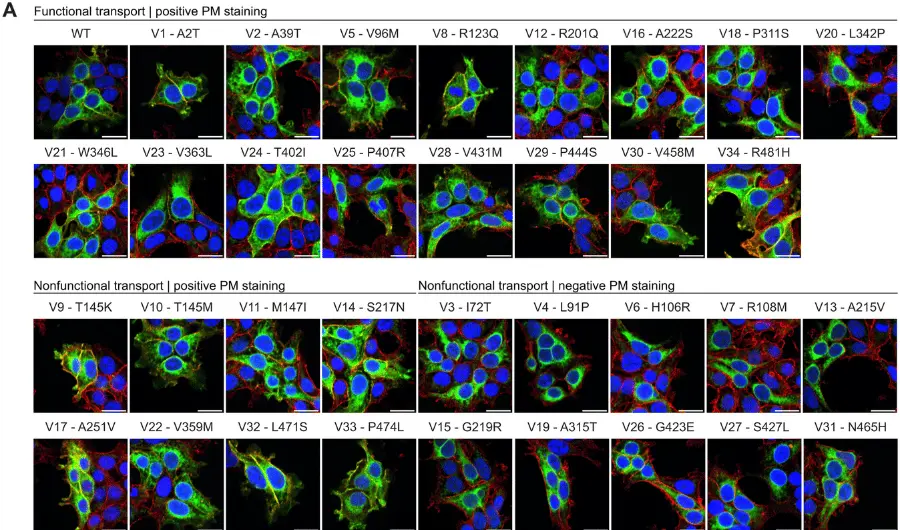
Brand Name to Generic Substitution of Levetiracetam in Patients with Epilepsy
July 2, 2018
PURPOSE: Levetiracetam is one of the most widely used antiepileptic drugs, but the evidence related to the safety of substitution from brand name to generic levetiracetam is scarce. The present study evaluated the risk of increased frequency of seizures after replacement of a brand-name levetiracetam with a generic product.
METHODS: We enrolled patients with epilepsy who were treated with branded levetiracetam for at least 6 months of sustained use. Patients were advised to switch to the generic levetiracetam. We analyzed data from 6 months before, to 6 months after, generic substitution. Increased seizure frequency was defined as a? 50% increase in seizure frequency after conversion date compared with seizure frequency before the conversion date. We analyzed changes in seizure frequency and performed subgroup analysis according to changes in seizure frequency.
RESULTS: We analyzed 148 epilepsy patients. Among the 148 patients, 109 (73.6%) were seizure-free before substitution and 105 patients remained seizure-free after switching. After generic substitution, an increased seizure frequency was noted in seven patients (4.7%), and a decreased seizure frequency was noted in 10 (6.8%). Patients with decreased seizure frequency were significantly younger (p?=?0.035) than those with an unchanged seizure frequency.
CONCLUSION: This study suggests that the risk of increased seizure frequency after generic substitution was minimal. The generic substitution of levetiracetam was generally safe, although larger prospective studies are warranted to corroborate our findings.