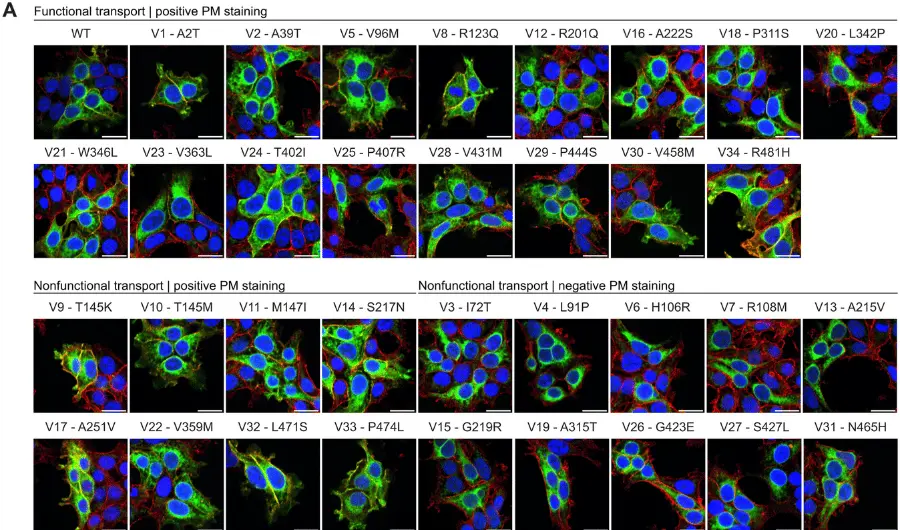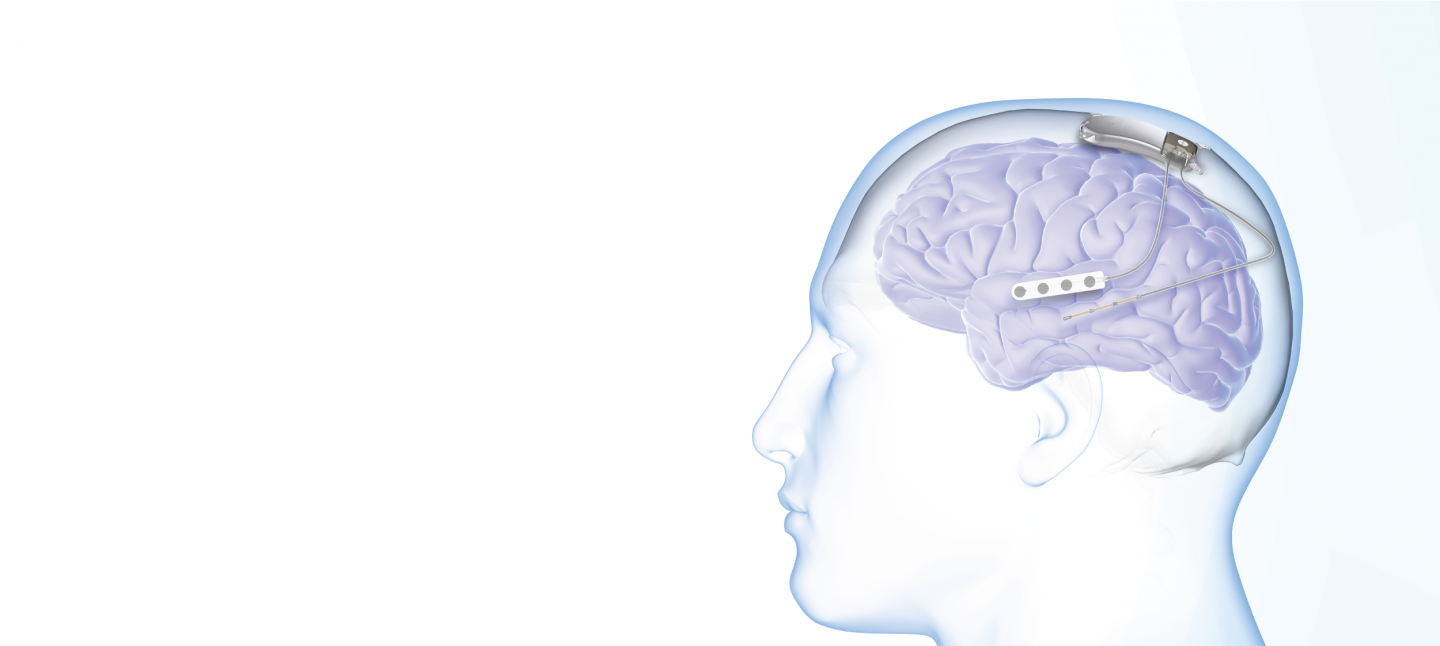
The Food and Drug Administration (FDA) has approved MRI labeling for the RNS® System (NeuroPace), a closed-loop brain-responsive neurostimulation system indicated as adjunctive therapy to reduce the frequency of seizures in adults with partial onset seizures who have undergone diagnostic testing that localized no more than 2 epileptogenic foci, are refractory to 2 or more antiepileptic medications, and currently have frequent and disabling seizures (motor partial seizures, complex partial seizures and / or secondarily generalized seizures).
With this approval, an MRI scan may be safely performed on patients with the RNS-320 neurostimulator model under the specific conditions of safe use detailed in the MRI Guidelines for the RNS System, providing an additional option for patients with focal onset seizures with brain anomalies that require frequent monitoring (ie, patients with tuberous sclerosis, brain tumors, and multiple sclerosis).
“MRI conditional labeling opens up valuable medical imaging possibilities for our patients treated with the model RNS-320 neurostimulator, who can now receive full-body 1.5T magnetic resonance imaging scans under appropriate conditions,” said Michael Favet, President and CEO of NeuroPace. “I’m pleased that we have removed a potential barrier to treatment and increased the number of patients who can benefit from this life-changing therapy.”