Study Reveals How Glial Cells May Play Key Epilepsy Role
April 30, 2019
A study provides potential new targets for treating epilepsy and novel fundamental insights into the relationship between neurons and their glial “helper” cells. In eLife, scientists at MIT’s Picower Institute for Learning and Memory report finding a key sequence of molecular events in which the genetic mutation in a fruit fly model of epilepsy leaves neurons vulnerable to becoming hyper activated by stress, leading to seizures.
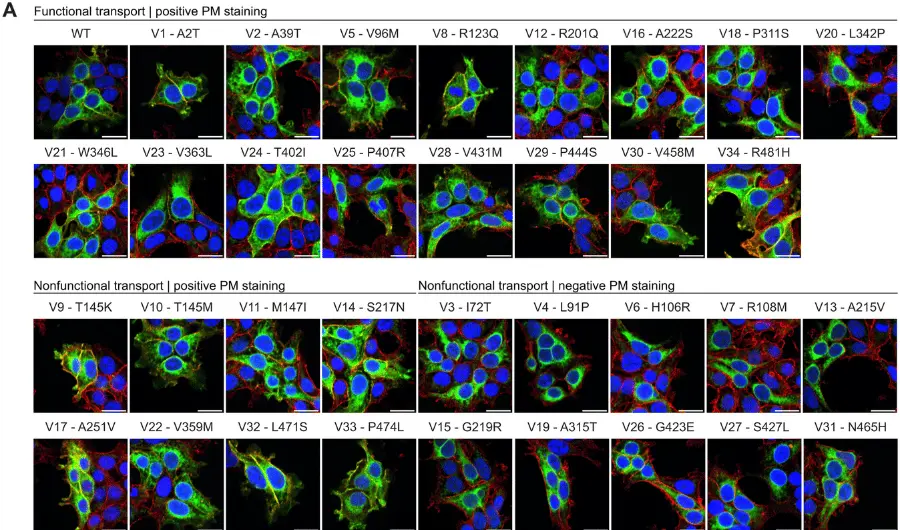
About 60 million people worldwide have epilepsy, a neurological condition characterized by seizures resulting from excessive neural activity. The “zydeco” model flies in the study experience seizures in a similar fashion. Since discovering zydeco, the lab of MIT neurobiologist Troy Littleton, Menicon Professor in Neuroscience, has been investigating why the flies’ zydeco mutation makes it a powerful model of epilepsy.
Heading into the study, the team led by postdoc Shirley Weiss knew that the zydeco mutation was specifically expressed by cortex glial cells and that the protein it makes helps to pump calcium ions out of the cells. But that didn’t explain much about why a glial cell’s difficulty maintaining a natural ebb and flow of calcium ions would lead adjacent neurons to become too active under seizure-inducing stresses such as fever-grade temperatures or the fly being jostled around.
The activity of neurons rises and falls based on the flow of ions – for a neuron to “fire,” for instance, it takes in sodium ions, and then to calm back down it releases potassium ions. But the ability of neurons to do that depends on there being a conducive balance of ions outside the cell. For instance, too much potassium outside makes it harder to get rid of potassium and calm down.
The need for an ion balance – and the way it is upset by the zydeco mutation – turned out to be the key to the new study. In a four-year series of experiments, Weiss, Littleton and their co-authors found that excess calcium in cortex glia cells causes them to hyper-activate a molecular pathway that leads them to withdraw many of the potassium channels that they typically deploy to remove potassium from around neurons. With too much potassium left around, neurons can’t calm down when they are excited, and seizures ensue.
“No one has really shown how calcium signaling in glia could directly communicate with this more classical role of glial cells in potassium buffering,” Littleton said. “So this is a really important discovery linking an observation that’s been found in glia for a long time – these calcium oscillations that no one really understood – to a real biological function in glial cells where it’s contributing to their ability to regulate ionic balance around neurons.”