Therapy That Targets Underlying Cause of Dravet Syndrome Gets Orphan Drug Status
August 8, 2019
The FDA has granted Orphan Drug designation to STK-001 (Stoke Therapeutics) for the treatment of Dravet syndrome.
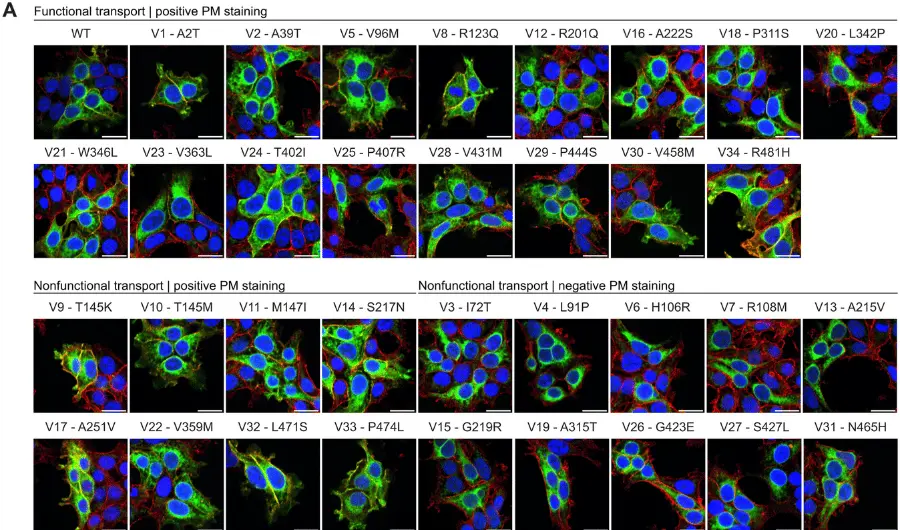
Dravet syndrome is a severe and progressive genetic epilepsy that starts within the first year of life and often leads to cognitive regression or developmental stagnation, ataxia, and speech impairment. The disease is characterized by frequent, prolonged and refractory seizures, in which approximately 85% of cases are due to a spontaneous, heterozygous loss of function mutations in the SCN1A gene.
STK-001 is an investigational antisense oligonucleotide that works by upregulating Nav1.1 protein expression from the non-mutant (wild type) copy of the SCN1A gene to restore physiological Nav1.1 levels. STK-001 is designed to address the underlying cause of Dravet syndrome, thereby reducing the occurrence of seizures and significant non-seizure comorbidities. The Company has generated preclinical data for STK-001 to demonstrate proof-of-mechanism.